The immune system has fixed and circulating parts. There are four major characteristics of the immune system:
1) The immune system can distinguish between SELF and NON-SELF tissue. This is important because it prevents the system from destroying itself.
2) The system is adaptive. It is not static but grows and changes with exposure and experience.
3) The system is highly specific. For example, an antibody can distinguish between a P on a molecule and an O substitute. This is both beneficial and bad in that it does not allow for cross protection. There are some notable exceptions to this precept; e.g. identification of smallpox vs. cowpox.
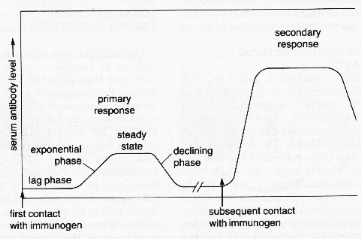
4) The system has memory. The second time an antigen is encountered, the immune system is faster and more effective. Ten (10) to fourteen (14) days after the primary exposure, the immune response peaks. Three days after secondary exposure, the immune response peaks.
 @ Dr Todd Hewitt
@ Dr Todd Hewitt
The immune system of humans consists of two parts. One part is called the HUMORAL IMMUNE SYSTEM because it involves circulating antibodies. These antibodies are produced by lymphocytes called
B-CELLS. The other part of the immune system is called CELL-MEDIATED. The cellular immune response depends on lymphocytes called T-CELLS which are located in both the blood and lymphatic tissues. To accomplish this type of protection, two populations of lymphocytes are produced.


GENETICS OF THE IMMUNE SYSTEM
(The following material is important in helping us understand how the immune system develops and functions. It is NOT testable.)
Molecules that mark a cell as SELF are encoded by a group of genes that is contained in a section of chromosome 6 in humans . This region is known as the major histocompatibility complex (MHC). The prefix "histo" means tissue; the MHC was discovered in the course of tissue transplantation experiments. Because MHC genes and the molecules they encode vary widely in the details of their structure from one individual to another (a diversity known as polymorphism), trans-plants are very likely to be identified as foreign by the immune system and thus be rejected. Scientists eventually discovered a more natural role for the MHC: it is essential to the immune defenses. MHC markers determine which antigens individuals can respond to, and how strongly. Moreover, MHC markers allow immune cells such as B-cells, T-cells and macrophages to recognize and communicate with each other. Thus, in humans, the MHC is responsible for: 1. graft rejection 2. controlling destruction of virus infected cells 3. regulating communication between cells of the immune system; this is probably its major role - cells must communicate or interact with each other 4. regulating the magnitude of the immune response 5. associated with susceptibility to or incidence of certain diseases; e.g. Multiple Sclerosis, Systemic Lupus Erythematosus, Myasthenia Gravis One group of proteins encoded by the genes of the MHC are the markers of self that appear on almost all body cells. Known as Class I MHC antigens, these molecules alert killer T cells to the presence of the body cells that have been changed for the worse - infected with a virus or transformed by cancer - and that need to be eliminated.< A second group of MHC proteins, Class II antigens, are found on B-cells, macrophages, and other cells responsible for present-ing foreign antigen to helper T cells. Class II products combine with particles of foreign antigen in a way that showcases the antigen and captures the attention of the helper T-cell. This focusing of T cell antigen recognition through class I and class II molecules is known as MHC (or histocompatibility) restriction. Not only do these markers distinguish between B and T cells, they distinguish among various subsets of cells that behave dif-ferently. Every mature T cell carries a marker known as T3 (or CD3); in addition, most helper T cells carry a T4 (CD4) marker, a molecule that recognizes class II MHC antigens. A molecule known as T8 (CD8), which recognizes class I MHC antigens, is found on many suppressor/cytotoxic T cells. In addition, different T cells have different kinds of antigen receptors - either alpha/beta (a /b) or gamma/delta (g/d).
Antibody Diversity
Scientists were long puzzled by the opulence of the immune system resources. The body apparently could recognize and mount unique responses to an endless variety of antigens - but how in the world could all the information be crammed into a limited number of genes?
The answer came as a surprise. A typical gene consists of a fixed segment of DNA, which directs the manufacture of a given protein molecule such as insulin. Antibody genes are assembled from bits and pieces of DNA scattered widely through the genetic material. As the B cell matures, it rearranges or shuffles these gene components, picking and choosing among hundreds of DNA seg-ments - some for each of the antibody's variable (V), diversity (D), joining (J), and constant (C) regions. Intervening segments of DNA are cut out by endonucleases; the selected pieces are spliced together by ligase enzymes.
The new gene - and the antibody it encodes - are virtually unique. When the B cell containing this uniquely rearranged set of gene segments proliferates, all its descendants will make this unique antibody. Then, as the cells continue to multiply, numerous mutants arise; these allow for the natural selection of antibodies that provide better and better "fits" for the target antigen. The result of this entire process is that a limited number of genetically distinct B cells can respond to a seemingly unlimited range of antigens. A similar mechanism was found to control a comparable structure on the T cell, the T cell antigen receptor. The variable regions of T cell antigen receptors, like those of antibodies, are encoded by the V, D, and J segments originally far apart, but which are brought together and fused into a single gene. With numerous candidates for each segment, the number of possible combinations becomes astronomical. In contrast to antibody genes, T cell receptor genes do not mutate as the T ccells proliferate. This insures that the self-tolerance imposed in the thymus will not be overthrown by the inadvertent generation of mutant T cell receptors that are anti-self.
Receptors for Recognizing Antigen
In order to recognize and respond to antigens that are their specific targets, both B and T cells carry special receptor molecules on their surface. For the B cell this receptor is a prototype of the antibody the B cell is prepared to manufacture, embedded in its surface membrane. When a B cell encounters a match-ing antigen in the blood or other body fluid, this antibody-like receptor allows the B cell to interact with it very efficiently.
 @ Dr. Todd Hewitt
@ Dr. Todd Hewitt
The T cell receptor is more complex. Structurally it is somewhat similar to an antibody, made of chemically linked chains with variable and constant regions. (But to work it needs the help of an associated set of signalling and anchoring cell sur-face molecules called T3.) Unlike a B cell, however, a T cell cannot recognize antigen in its natural state; the antigen must first be broken down, and the fragments bound to a MHC molecule, by an antigen presenting cell.(note Figure 16.23)
Helper T cells (T4 cells) look for antigen bound to a class II MHC molecule - a combination displayed by macrophages and B cells. Now you can appreciate the damage done by the HIV virus.
The T4 (CD4) receptor molecule serves as a receptor for the HIV virus. Hence, this functionally critical protein for helper T cells is a gate through which HIV enters a human system, leading to AIDS. It destroys the T4 helper cells and thus prevents the final destruction of parasites that have been ingested by macrophages or attached by B cells. Without the linkage between the B and T4 cells, the B cells do not proliferate and produce large numbers of Ab against the invaders. Most cytotoxic T cells (T8 cells), on the other hand, respond to antigen bound to MHC class I molecules which are found on almost all body cells.
The T cell receptor molecule forms a three-way complex with its specific foreign antigen and an MHC protein. This complicated arrangement ensures that T cells - which affect other cells through either direct contact or bursts of secretions - act only on precise targets and at close range.
The major antigen receptor, named alpha/beta for its two chains, is found on most T4 and T8 cells. A second, more recently discovered antigen receptor also has two chains and is known as gamma/delta. It is found on a distinct subset of mature T cells. Like the alpha/beta receptor, the more primitive gamma/delta receptor works in conjunction with T3. The function of T cells that carry gamma/delta receptors is not known at present.
 @ Dr. Todd Hewitt
@ Dr. Todd Hewitt
Genetics and Transplants
The success of a transplant - acceptance or rejection - depends on the stubbornness of the immune system. For a transplant to "take," the body of the recipient must be made to suppress its natural tendency to get rid of foreign tissue.
Scientists have tackled this problem in two ways. The first is to make sure that the tissue of the donor and the recipient are as similar as possible. Tissue typing, or histocompatibility testing, involves matching the markers of self on body tissues.
Because the typing is usually done on white blood cells or leukocytes, the markers are referred to as human leukocyte antigens (HLA). Each cell has a double set of six major antigens, designa-ted HLA-A, B, C, and three types of HLA-D -- DR, DP, and DQ. (HLA-A, B, and C are the same as the class I antigens encoded by the genes of the major histocompatibility complex. HLA-D region molecules are the class II MHC antigens.
Each of the HLA antigens exists - in different individuals - in as many as 20 varieties, so that the number of possible HLA types reaches about 10,000. Histocompatibility testing relies on antibodies to determine if a potential organ donor and recipient share two or more HLA antigens, and thus are likely to make a good "match." The best matches are identical twins; next best are close relatives, especially brothers and sisters.
MHC and Disease AssociationHLA
1. Ankylosing spondylitis; B-27
2. Juvenile rheumatoid arthritis; B-27 & DR-4
3. Juvenile diabetes; B-8 & DR-3
4. Myasthenia gravis; B-8
5. Ragweed allergy; DR-2
6. Multiple sclerosis; DR-2
7. SLE (Systematic Lupus Erythematosus; DR-3 & DR-2
The second approach to taming rejection is to lull the recipient's immune system. This can be achieved through a variety of powerful immunosuppressive drugs. Steroids suppress lymphocyte function. The drug cyclosporine holds down the production of the lymphokine interleukin-2, which is necessary for T cell growth. When such measures fail, the graft may yet be saved with a new treatment. OKT3 is a monoclonal antibody (see related information on monoclonal antibodies) that seeks out the T3 marker carried on all mature T cells. By either destroying T cells or incapacita-ting them, OKT3 can bring an acute rejection crisis to a halt.
Not surprisingly, any such all-out assault on the immune system leaves a transplant recipient susceptible to both oppor-tunistic infections and lymphomas. Although such patients need careful medical follow-up, many of them are able to lead active and essentially normal lives.
There are four (4) functional T cell subpopulations which have been recognized and named according to their associated activity. Two types of T-lymphocytes are called EFFECTOR CELLS because they are responsible for CMI. These are the (1) cytotoxic T cells which disrupt target cells by direct cell-to-cell contact. These are responsible for graft rejection and also kill virus infected cells. They are important in controlling the Herpes virus. These cells will also kill tumor cells. (2) Delayed hypersensitivity cells which bring about inflammation and activate macrophages in delayed hypersensitivity reactions. The other two types are called REGULATOR CELLS because they oversee the immune response. They are (3) the T helper cell which work with B cells in antibody production. These control all immune responses and self vs. non-self immunities. The AIDS virus kills the helper T cells. (4) T suppressor cells which act to regulate or suppress the activities of other types of T and B cells. These cells aid in homeostatic balance between Ab production and repression. Basically the role of T-cells is two-fold:
1. Regulation - Helper T increase magnitude of response - Suppressor T decrease (turn off) response 2. Cytotoxicity - kill tumor cells, virus infected cells, - foreign tissue rejection (grafts, transplants)T cells with specific receptors for a given antigenic determinant transform into lymphoblast cells on exposure to the immunogen. Lymphoblast cells develop into sensitized T lymphocytes and memory cells (see diagram below). Sensitized t-lymphocytes may directly deal with antigen (cytotoxic lymphocytes), or they may release lymphokines. Lymphokines are produced in minute quantities, act locally in the host, and are not specific for the antigen. They are named according to their biological activity - over 100 have been named to date. Below are a few examples.

Lymphocyte Mediators - Lymphokines
Mediators affecting Macrophages
Migration inhibitory factor (MIF)
Macrophage-activating factor (MAF) indistinguishable from MIF
Chemotactic factors for macrophages
Antigen-dependent MIF
Mediators affecting polymorphonuclear (PMN) leukocytes
Chemotactic factors
Leukocyte inhibitory factor (LIF)
Eosinophil stimulation promoter (ESP)
Mediators affecting Lymphocytes
Mitogenic factors such as Interleukin-2 which stimulate growth
and division of T-cells
Factors enhancing antibody formation
Factors suppressing antibody formation
Mediators affecting other cells
Cytotoxic factors, Lymphotoxin (LT)
Growth inhibitory factors (? same as LT)
Osteoclastic factors (OAF)
Collagen-producing factor
Colony stimulating factor
Interferon
Immunoglobulin-binding factor (IBF)
Procoagulant (tissue factor)
T Cells and their Immune Products
MACROPHAGE-PROCESSED IMMUNOGEN
¯
T-cell
¯
Lymphoblast cell
¯ ø
¯ ø
¯ MEMORY CELLS
¯
Sensitized (Activated) Lymphocytes
÷ ¯
÷ ¯
Cytotoxic Lymphocytes ¯
¯
¯
-----------------LYMPHOKINES-----------------
¯ ÷ ¯ ø ¯
¯ ÷ ¯ ø ¯
¯ ÷ INTERFERON ø ¯
¯ TRANSFER FACTOR ø Other Lymphokines
¯ ø Chemotactic factor
¯ ø for Neutrophils &
¯ ø Eosinophils; ¯ ø Osteoclast-acti-
¯ ø vating factor
¯ ø
MITOGENIC FACTOR for ø
non-committed Lymphocytes MACROPHAGE CHEMOTACTIC FACTOR ¯ MACROPHAGE INHIBITING FACTOR
¯ MACROPHAGE ACTIVATING FACTOR
¯
LYMPHOKINES
Responses of immunogen-exposed T cell
Osteoclasts have been shown to be fixed macrophages of the skeletal system.
Simplified Version of Immune System DevelopmentBONE MARROW -------------------® LYMPHOID STEM CELLS
÷ ø
÷ ø
T CELL PRECURSOR B CELL PRECURSOR
(BLAST CELL) (BLAST CELL)
¯ ¯
¯ ¯
THYMUS GLAND BURSA OF FABRICUS
¯ IN BIRDS; SPLEEN
¯ AND GUT CELLS IN
¯ HUMANS (G.A.L.T)
¯ ¯
THYMOSINS ¯
¯ ¯
¯ ¯
MATURE T CELL <-------> MATURE B CELL
¯ ¯
¯ ¯
CELL-MEDIATED Ab Production
IMMUNITY (Circulating
(Lymph Nodes, Immunity)
Tonsils, Spleen)
Thymus Gland - secretions of this gland process T lymphocyte blast cells and ultimately determine the development of cell mediated immunity. THYMOSINS - peptides secreted by the thymus; some function as true hormones, some as immune regulators; there are two general categories: Alpha and Beta Some Examples: Thymosin Alpha-1 - potent inducer of helper T cells Alpha-7 - induces suppressor T cells Thymopoetin - influences T cell differentiation Beta Thymosins alter levels of other hormones Thymosin Beta-4 - stimulates anterior pituitary to release LH (luteinizing hormone which stimulates ovulation) Other Thymosins affect corticosteroid hormones
 Back to Homepage
Back to Homepage Back to Menu Page
Back to Menu Page Back to Microbiology Start Page
Back to Microbiology Start Page