The Bohr model
The Bohr Model is probably familar as the "planetary model" of the atom illustrated in the adjacent figure that, for example, is used as a symbol for atomic energy (a bit of a misnomer, since the energy in "atomic energy" is actually the energy of the nucleus, rather than the entire atom). In the Bohr Model the neutrons and protons (symbolized by red and blue balls in the adjacent image) occupy a dense central region called the nucleus, and the electrons orbit the nucleus much like planets orbiting the Sun (but the orbits are not confined to a plane as is approximately true in the Solar System). The adjacent image is not to scale since in the realistic case the radius of the nucleus is about 100,000 times smaller than the radius of the entire atom, and as far as we can tell electrons are point particles without a physical extent.
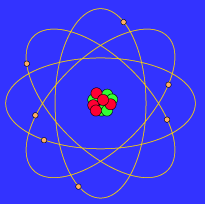
This similarity between a planetary model and the Bohr Model of the atom ultimately arises because the attractive gravitational force in a solar system and the attractive Coulomb (electrical) force between the positively charged nucleus and the negatively charged electrons in an atom are mathematically of the same form. (The form is the same, but the intrinsic strength of the Coulomb interaction is much larger than that of the gravitational interaction; in addition, there are positive and negative electrical charges so the Coulomb interaction can be either attractive or repulsive, but gravitation is always attractive in our present Universe.)
Back
Electron Configuration
