The Nucleus

Nuclear Stability and Radioactive Decay
The nucleus of a radioisotope is unstable. In an attempt to reach a more stable arrangement of its protons and neutrons, the nucleus will spontaneously decompose to form a different nucleus. If the number of neutrons changes in the process, a different isotopes is formed. If the number of protons changes in the process, then an atom of a different element is formed. This decomposition of the nucleus is referred to as radioactive decay. During radioactive decay an unstable nucleus spontaneosly decomposes to form a different nucleus, giving off radiation in the form of atomic partices or high energy rays. This decay occurs at a constant, predictable rate that is referred to as half-life. A stable nucleus will not undergo this kind of decay and is thus non-radioactive.
The Kinetics of Radioactive Decay
A radioactive nuclide may undergo a number of decay events, called a decay series, until a stable nuclide is formed, Radioactive decay follows first-order kinetics. The half-life of a radioactive sample is the time required for the amount of the nuclide to reach half the original amount. The transuranium elements, those coming after uranium in the periodic table, can be synthesized by particle bombardment of uranium of heavier elements.
Nuclear Transformations
Nuclear transformations are anything that changes from one isotope to another, or comnies two or more to form another is a transformation.
Detection and Uses of Radioactivity
There are many ways in which one can detect radioactivity. Some methods include using the Geiger-Müller tube, Photographic Film, Gold Leaf Electroscope, Spark Counter, Cloud Chamber & Bubble Chamber, Modern Detectors. Some uses of radioactivity include smoke detectors, sterilizing, radioactive dating, and most importantly, cancer treatment.

Thermodynamic Stability of the Nucleus
The Thermodynamic stability of the nucleus involved a comparison of the energy of the nucleus to that of of its components nucleons. When a system gains or loses energy, it also gains of loses a quantity or mass, given by the relationship E=MC(squared). The change in mass, called the mass defect, can be obtained by comparing the nuclear mass to the sum of the masses of the component nucleons and can be used to calculate the energy of formation of a nucleus form its nucleons. The binding energy is the energy required to decompose the nucleus into protons and neutrons.
Nuclear Fission and Fusion

Fission is a nuclear reaction in which an atomic nucleus splits, or fissions, into fragments, usually two fragments of comparable mass, with the release of large amounts of energy in the form of heat and radiation. In the UWNR, Uranium-235 is the fuel and it is struck by a moving neutron, which combines with the U-235 to become U-236. Because of the mass and energy imparted to the nucleus by the neutron, the nucleus has enough energy to fission and breaks down into two (or more) smaller nuclei and two or three new neutrons which together have less mass than the original U-236 nucleus. This missing mass, sometimes known as the mass defect, is changed into energy.

Energy
can also be produced by combining light nuclei in a process is called nuclear
fusion. As an energy source, fusion has several advantages over fission: the
light nuclei are plentiful and easy to obtain, and the end products of fusion
are usually light, stable nuclei rather than heavy radioactive ones. There is
one considerable disadvantage: before light nuclei can be combined however,
their mutual repulsion must be overcome due to the fact that the positively
charged protons of the nuclei repulse each other. Because of this problem,
fusion reactors are not yet producing electrical power. This is an area of great
research interest in the field of nuclear engineering and physics.
Effects of Radiation
This was taken from CNN. To see the entire article, click here.
Exposure to radiation can have a dramatic and immediate effect on the human body.
The gastrointestinal system is very sensitive to radiation, leading to nausea and vomiting immediately after exposure. The blood system is often the hardest hit, although antibiotics and transfusions may allow a recovery.
But severe radiation damage to the immune system can cause overwhelming infections. And although nerves and the brain are most resistant to radiation, acute exposure usually results in damage to the central nervous system. High doses can kill outright.
|
||||||||||||||||||||||||||||
The long-term effects of radiation exposure can include sterility, cancer and genetic damage that can be passed to children.
However, experts say there are three ways to minimize the risk of radiation exposure:
As officials in Japan cope with radiation exposure from Thursday's accident, they will also try to avoid some of the mistakes made 13 years ago when a serious accident occurred at the Chernobyl nuclear power plant in Ukraine.
People in the affected area were actually told to gather outside, despite high levels of radiation still coming from the plant. The pictures were part of an effort by the Soviet government, then in power, to convince the outside world that all was well.
Thousands of cases of thyroid cancer and leukemia resulted.
By contrast, in Japan, people in the area near the accident were being ordered to stay inside their homes, to keep them shielded from potential radiation.
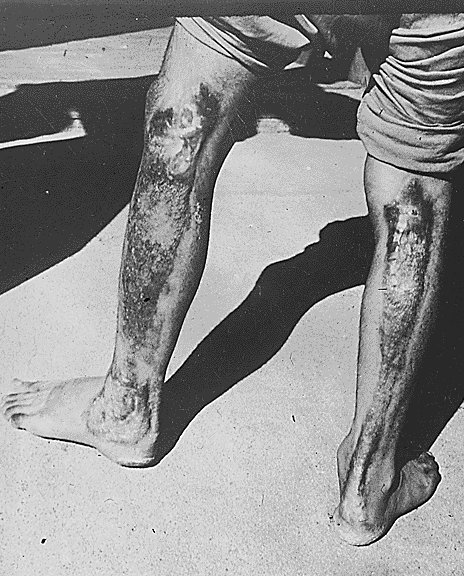 |
This is a picture of a man who is diagnosed with excessive radiation. This was a result of the Hiroshima Nuclear Bombing. |