Organic Chemistry
Alkanes: Saturated Hydrocarbons

Alkanes
are saturated hydrocarbons. As the name indicates, hydrocarbons are
compounds composed of carbon and hydrogen.
Those carbons whose carbon-carbon bonds are single bonds are said to be
saturated, because each carbon is bound to four atoms, the maximum number. Hydrocarbons containing carbon-carbon multiple bonds are
described as being unsaturated, since the carbon atoms involved in a multiple
bond can react with additional atoms.
The simplest member of the saturated hydrocarbons is methane. Methane is a
tetrahedral structure and can be described in terms of a carbon atom using a
hybrid set of orbitals to bond to the four hydrogen atoms.
The next alkane, the one containing two carbon atoms, is ethane. Each
carbon in ethane is surrounded by four atoms and thus adopts a tetrahedral
arrangement, as expected from the localized electron model.
Because they are saturated compounds and because the C-C and C-H bonds are
relatively strong, the alkanes are fairly unreactive. They do not react
with acids, bases, or strong oxidizing agents. This chemical inertness
makes them valuable as lubricating materials and as the backbone for structural
materials such as plastics.
At a sufficiently high temperature, alkanes do react vigorously and
exothermically with oxygen, and these combustion reactions are the basis for
their widespread use as fuels. The
alkanes can also undergo substitution reactions, primarily where halogen atoms
replace hydrogen atoms.
Substances such as chlorine or fluorine in methane as a substitution are very
unreactive and due to their chemical inertness, stay in the earths atmosphere
for a prolonged period of time. Since they are there for so long and their
altitude is increasing, soon enough they reach altitudes where they may be a
threat to our protective ozone layer.
Alkenes and Alkynes
First,
it would be necessary to define what alkenes and alkynes are.
Both are part of the family of hydrocarbons.
However, in the same way that they are similar, they also differ.
Alkenes are hydrocarbons that have the general formula C H and contain a
carbon-carbon double bond. Alkynes
are also hydrocarbons. They are
classified as unsaturated hydrocarbons, which contain a carbon-carbon triple
bond. Unsaturated hydrocarbons are
hydrocarbons that do not contain the maximum number to hydrogen atoms for a give
carbon-atom framework.
The
simplest form of an alkene is called ethylene, and its formula is C H.
The simpolest alkyne is known as ethyne, which is C H.
The nomenclature of alkenes is very similar to that of alkanes, however
the ending -ene is used instead of -ane. The
same goes for alkynes where the name is ended with -yne.
Both
alkanes and alkynes important reactions, such as addition reactions and
hydrogenation reactions. Hydrogenation
of alkenes is used to industries to make solid shortenings, in which unsaturated
dfats alter to solid saturated fats. Halogenation is also a type of reaction.
Both hydrogenation and halogenation fall under the same category of
addition reactions. Where as
hydrogenation is the addition of hydrogen atoms, halogenation is the addition of
halogen atoms. Polymerization is
also a reaction that occurs in certain unsaturated hydrocarbons.
In this case, small molecules bind to make larger molecules.
Aromatic Hydrocarbons
Aromatic compounds have distinctive odors. A couple of example include benzene and the allay derivatives of benzene. Benzene is a six-sided ring drawn with alternating single and double bonds. The molecular formula for the Benzene Ring is C6H6 and the structure
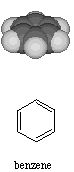
is
hexagon shaped with a carbon atom at each corner.
It is quite important, due to the fact that any compound with this ring is an
aromatic compound. Note, however,
that "aromatic" hydrocarbons sometimes are odorless.
The term "aromatic" came to be used because earlier compounds
found with rings had pleasant fragrances. Now,
it turns out that the Benzene ring has nothing to do with smell.
For instance, asprin is an aromatic compound.
Yet, aspirin is odorless.
The carbon
atoms are all connected by hybrid partial double bonds. They are not connected by alternating single or double bonds.
Overlapping "p" orbitals cause "delocalization" of
their bonds. This gives stability
to the ring system. If more
reactions were to take place, they would cause the break of stabilization. Instead, benzene refers to react by substitution.
Therefore, the ring is very stable.
Benzene is
synthesized from petroleum and thousands of compounds are derived from benzene.
Aromatic hydrocarbons are found in several things, including TNT,
rubbers, and mothballs.
The Petrochemical Industry

The word Petrochemical is a broad definition; they range from gasoline to aromatics. Petrochemicals in various forms had been known since the mid-nineteenth century. Before the petrochemical energy solution there was a variety of coal based products. Because of the availability and low cost of coal, it was ensured to prevail as an energy source. At about the same time though, oil and natural gas were becoming increasingly available and scientists were beginning to examine them as energy alternatives to coal. In the late 1850s, rock oil, as petroleum was called, was being tested as an illuminator to replace the much used but highly flammable camphene. Kerosene came into play at about the same time but it was most likely carbon black of the tire industry that was the first major petrochemical. The abundance of natural gas also led to its use as a feedstock to produce glycols, esthers, amines, and ketones.
The petrochemical industry grew to its status as one of the most important enterprises in the global network of business in a matter of a few decades. Born in the early 1920s, the petrochemical industry continued to grow throughout the 40s, 50s, and 60s until its peak in the 1970s. This period gave birth to some of the largest chemical companies in the United States including Exxon Chemical, Oxychem, and ARCO chemical. These companies, along with pioneers such as Vladimir Haensel and Eugene Houdry allowed the American chemical industry to exceed that of Europe and secured its place as a major player in the world wide chemical industry. Although the international petrochemical industry expanded in the 1970s, causing increased competition, the domestic industry has made a comeback in recent years.
With the potential in these early petrochemicals, along with the increasing availability of petroleum and natural gas, the industry grew rapidly in the 20s and 30s. This is about the time that today's major petrochemical companies entered the field. As more and more pioneering companies such as Standard oil and Exxon entered the field, competition was increased. The future of organic chemicals in the U.S. and the shift from coal to oil was becoming much more defined. World War II brought new prospects to the industry, providing new sector such as aviation fuel and synthetic rubber. After the war the plastics industry was the primary expansion in the petrochemical industry. In the 1950s, American companies began to venture into the international market and within two decades petrochemicals became the largest sector in the American economy. After its peak though, the industry began to experience a downfall. The uprising of environmentalism in the late 60s and the generally negative views of the chemical industry fueled the downfall. Other elements such as foreign competition, and economic recession, took the edge off America's advantage. Although the industry had fallen, it was not completely wiped out. In the 80s and 90s, restructuring of the industry led to rebirth and the new companies merged to form today's big companies such as Shell and Mobile.
Hydrocarbon
Derivatives
Hydrocarbon
derivatives are molecules that are fundamentally hydrocarbons, but have
additional atoms or groups of atoms called functional groups.
Hydrocarbon derivatives are primarily made up of Alcohols, Aldehydes and
Ketones, Carboxylic Acids and Esters, and Amines.
Alcohols are characterized by the presence of the hydroxyl group (-OH).
The systemic name for an alcohol is obtained by replacing the final –e
of the parent hydrocarbon by –ol. Alcohols
usually have much higher boiling points than might be expected from their molar
masses. Although there are many
important alcohols, the simplest ones, methanol and ethanol, have the greatest
commercial value. Methanol is used
as a starting material for the synthesis of acetic acid and many types of
adhesives. Fibers.
And plastics. It also can be
used as a motor fuel. Methanol is
highly toxic to humans and can lead to blindness and death if ingested.
Ethanol is the alcohol found in beverages such as beer, wine, and
whiskey.

Aldehydes
and Ketones contain the carbonyl group. The
carbonyl group is one type of double bond. The unique properties and reactivity of Aldehydes and Ketones
arise from their unique charge distribution.
The systemic name for aldehyde is obtained from the parent alkane by
removing the final –e and adding –al. For ketones, the final –one replaces -e, and the number
indicates the position of the carbonyl group where necessary.
Ketones often have useful solvent properties and are frequently used in
industry for this purpose. Aldehydes and ketones are most often produced commercially by
the oxidation of alcohols. Carboxylic
acids and esters are characterized by the presence of the carboxyl group and
have the general formula RCOOH. These
molecules are typically weak acids in aqueous solutions.
Organic acids are named from the parent alkane by dropping the final –e
and adding –oic. Many carboxylic
acids are synthesized by oxidizing primary alcohols with a strong oxidizing
agent. A carboxylic acid reacts
with an alcohol to form an ester and a water molecule.
Esters often have a sweet, fruity odor that s in contrast to the
often-pungent odors of the parent carboxylic acids.
Amines are probably best viewed as derivatives of ammonia in which one or more N-H bonds are replaced by N-C bonds. The resulting amines are classified as primary if one N-C bond is present, secondary is they contain two N-C bonds, and tertiary if all three N-H bonds in NH3 have been replaced by N-C bonds. Many amines have unpleasant fish-like odors.

A
picture of an AMINE factory.
Polymers
Polymers are everywhere and are part of everyone's life. Polymers are long, chainlike molecules. A polymer is made up of smaller molecules called monomers. The polymer polyethylene, for example is built from ethylene monomers, which are connected to each other. Polymers make up materials such as plastics and rubbers that we use everyday.
Polymers,
in some form, are present in every day life.
The production and manufacturing of polymers is vital to and prevalent in
our standard of living. The first
synthetic polymers were formed accidentally.
They were simply by-products of organic research and experiments.
Over the years, chemists began to discover uses for these polymers and
their industry grew. This growth is obvious today.
Polymers
are used in countless ways. They
can be found in nature as well as in the home.
Teflon, for example is used today in frying pans and engine treatments.
Polymers make all sorts of plastic.

This diaper containes polymers, which help to absorb liquid.
The Polymer Industry
Approximately
50% of the industrial chemists in the US work in some area of the polymer
chemistry. This shows how important polymers are to our economy and
standard of living. Polymers are essential to the production of goods.
These goods include anything ranging from toys to roofing materials. The
development of the polymer industry is a great example of the importance to
serendipity in the progress of science. Discoveries in the polymer
industry often arise from accidental observations. One example is that of
plastic. It can be traced back to 1846 when Christian Schoenbien spilled a
flask containing nitric and sulfuric acids. The first synthetic polymers
were obtained as by-products of various organic reactions. They were
generally regarded as unwanted contaminants.
Some
polymers include thermoset polymers where when molded to a certain shape under
pressure and high temperatures, can't be softened again or dissolved.
Another type is a thermoplastic polymer, where it can be remelted after it has
molded. Also, Elastomers are stretchable polymers. They are
materials that recover their shape after a deforming force. One chemist
who contributed greatly to the understanding of polymers was Wallace C.
Carothers. One of his accomplishments was the preparation of nylon.
The nylon story further shows the importance of serendipity in scientific
research.
A German chemical company discovered PVC, a high-volume polymer in 1912.
PVC can be used for clothing, upholstery, automobile bumpers, credit cards, and
packaging materials. It can also be used for pipes, floor, wall and window
coverings and more.