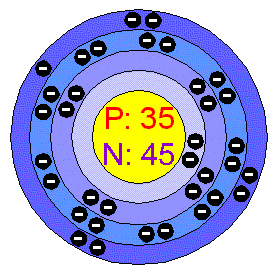
Bromine
Electronic Configuration
[Ar] .3d10 .4s2 .4p5
Reaction
Bromine, Br2 is not reactive with oxygen, O2, or nitrogen, N2. However, bromine does react with ozone, O3, the second allotrope of oxygen, at -78°C to form the dioxide bromine(IV) oxide, BrO2.
Br2(l) + 2O3(g) ------> 2BrO2(s) + O2(g)
Ion Charge
-1
Compounds
BrF- Bromine and Flouride
BrCl- Bromine and Chloride
IBr- Iodine and Bromine
HBr- Bromine and Thydrogen